Phenolic hydroxyl groups and antioxidant activity. Oxidation reaction of alcohols to aldehydes Reactions at the hydroxyl group
Phenolic hydroxyl is a hydroxyl bound to an aromatic radical. It contains drugs from the phenol group (phenol, resorcinol); phenolic acids and their derivatives (salicylic acid, phenyl salicylate, salicylamide, oxafenamide); phenanthrene isoquinoline derivatives (morphine hydrochloride, apomorphine); sinestrol, adrenaline, etc.
The chemical properties of compounds containing phenolic hydroxyl are determined by the interaction of an electron pair with the π-electrons of the aromatic ring. This interaction leads to a shift in the electron density from the OH group to the ring, disruption of the uniform distribution of electrons in it, and the creation of an excess negative charge in the ortho ( O)- and pair ( P)-positions. The hydrogen atom of the hydroxy group ionizes and gives phenols weak acidic properties (pKa of phenol = 10.0; pKa of resorcinol = 9.44). Therefore, unlike alcohols, they form salts with alkalis (at pH 12-13), soluble complex compounds with iron (III) chloride (in neutral, slightly alkaline and acidic solutions).
Phenols exhibit strong reducing properties and are very easily oxidized even by weak oxidizing agents. They form colored compounds with a quinoid structure.
The reactions of electrophilic substitution of hydrogens in O- And P-positions of the aromatic ring – halogenation (bromination), condensation with aldehydes, nitration, combination with diazonium salts.
Based on the properties of the phenolic hydroxyl and the aromatic ring activated by it, the following reactions are used in drug analysis:
1 – complex formation;
2 – halogenation (bromination);
3 – azo combinations;
4 – oxidation;
5 – formation of indophenol dye;
6 – condensation with aldehydes.
Identification
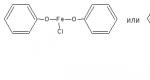
2.1. Complexation reaction with iron ions (III)
It is based on the properties of phenolic hydroxyl to form soluble complex compounds, often colored blue (phenol) or violet (resorcinol, salicylic acid), less often red (PAS - sodium) and green (quinosol, adrenaline).
The composition of the complexes, and, consequently, their color is determined by the amount of phenolic hydroxyls, the influence of other functional groups, and the reaction of the environment.
If there is an excess of phenol:

Presumable composition of the final product in the reaction with phenol:


2.2. Aromatic ring bromination reaction
Based on electrophilic substitution of hydrogen in O- And P- positions on bromine with the formation of an insoluble bromine derivative (white precipitate).

Basic rules for bromination:
Bromine replaces hydrogen in O- And P- positions relative to phenolic hydroxyl (the most reactive is P- position):

If available in O- or P- positions of the aromatic ring of substituents, fewer bromine atoms react;
If in O- or P- positions there is a carboxyl group, then in the presence of excess bromine, decarboxylation occurs and the formation of a tribromo derivative:

If the deputy is in m- position, then it does not interfere with the formation of the tribromo derivative:

If a compound contains two phenolic hydroxyls in m- position, then as a result of their coordinated orientation a tribromo derivative is formed:

If two hydroxyl groups are located in O- or P- positions to each other, they act inconsistently: bromination does not occur quantitatively:

If, in addition to phenolic hydroxyls, the compound contains an amide or ester group (salicylamide, phenyl salicylate), preliminary hydrolysis is necessary for their quantitative assessment by bromatometry.
2.3. Azo coupling reaction
The combination also goes to O- And P- provisions, in this case, as well as with bromination, it is preferable P- position. Diazo reagent is diazonium salt (diazotized sulfanilic acid). The environment is alkaline. The reaction product is an azo dye.

2.4. Oxidation reaction
Phenols can be oxidized to various compounds, but most often to O- or P-quinones (cyclic diketones), colored pink or, less commonly, yellow.


2.5. Reaction of formation of indophenol dye
It is based on the oxidation of phenols to quinones, which, when condensed with ammonia or an amino derivative and an excess of phenol, form an indophenol dye, colored violet.


A variation of this reaction is the Lieberman nitro reaction; it is characteristic of phenols that do not have substituents in O- And P- provisions.
When exposed to sodium nitrite in an acidic environment, it forms P-nitrosophenol, isomerizing to P- quinoidoxime, which, reacting with excess phenol in an acidic environment, forms indophenol:

2.6. Formation of nitroso compounds
When interacting with dilute nitric acid, phenols can be nitrated at room temperature, forming O- And P- nitro derivatives. The resulting nitro derivative contains P- position of the mobile hydrogen atom of the hydroxyl group, a tautomeric aci form with a quinoid structure is formed; it is usually colored yellow. The addition of alkali enhances the color due to the formation of a well-dissociated salt:

2.7. Condensation reaction with aldehydes or acid anhydrides
With formaldehyde in the presence of concentrated sulfuric acid to form an auric (arylmethane) dye colored red.
The reaction is pharmacopoeial for salicylic acid. Concentrated sulfuric acid plays the role of a water-removing agent in the first stage of the reaction, and acts as an oxidizing agent in the second.
With phthalic anhydride (fusion and subsequent dissolution of the melt in alkali) is recommended by the pharmacopoeia for the identification of phenol and resorcinol.

quantitation
2.8. Bromatometry
The method is based on the electrophilic substitution of hydrogen atoms of the aromatic ring with bromine, isolated in the reaction of potassium bromate with potassium bromide in an acidic environment.
K  BrO 3 + 5KBr + 6 HCl → 3Br 2 + 6KCl + 3H 2 O
BrO 3 + 5KBr + 6 HCl → 3Br 2 + 6KCl + 3H 2 O
Direct and reverse titration methods are used. Directly - titrate with potassium bromate in the presence of potassium bromide with a methyl orange or methyl red indicator from pink to discolored. At the equivalence point, an excess drop of potassium bromate releases bromine, which oxidizes the indicator and the solution becomes colorless. During back titration, excess potassium bromate is introduced, potassium bromide is added, an acidic environment is created, the time required for bromination is maintained, and then the excess bromine is determined iodometrically (starch as an indicator).
Br 2 + 2KI → I 2 + 2KBr
I 2 + 2Na 2 S 2 O 3 → Na 2 S 4 O 6 + 2NaI
By direct titration, thymol is determined by GF, and phenol, resorcinol, salicylic acid, synestrol and other drugs are determined by reverse titration.
M.e. = ¼ M.m. (thymol)
M.e. = 1/6 M.m. (phenol, resorcinol, salicylic acid)
M.e. = 1/8 M.m. (sinestrol)
2.9. Iodometry
Based on the electrophilic substitution of hydrogen atoms of the aromatic ring with iodine.

To bind hydroiodic acid, which shifts the equilibrium in the opposite direction, sodium acetate or sodium bicarbonate is added.
HI + NaHCO 3 → NaI + H 2 O + CO 2
HI + CH 3 COONa → NaI + CH 3 COOH
Direct and reverse titration methods are used. In the latter, excess iodine is titrated with sodium thiosulfate.
I 2 + 2NaS 2 O 3 → 2NaI + Na 2 S 4 O 6
M.e. = 1/6 M.m. (phenol)
2.10. Iodine chlorometry
The method is based on the electrophilic substitution of hydrogen atoms of the aromatic ring with iodine, which is part of iodine monochloride.

A back titration method is used - the excess of iodine monochloride is determined iodometrically.
ICl + KI → I 2 + KCl
I 2 + 2Na 2 S 2 O 6 → 2NaI + Na 2 S 4 O 6
M.e. = 1/6 M.m. (phenol)
2.11. Acetylation method
Used according to GF X for quantitative assessment of sinestrol.

M.e. = ½ M.m.
2.12. Alkalimetric method of neutralization in the protophilic solvent dimethylformamide (DMF).
The drug groups of phenols exhibit very weak acidic properties; their determination by the alkalimetric method of neutralization in aqueous or mixed media is impossible, therefore titration is used in a medium of non-aqueous solvents, in particular DMF. The method is based on the salt formation of a determined weak acid (phenol) with a titrant (sodium methylate) in a protophilic solvent that enhances acidic properties.

Total:

2.13. Photocolorimetry (FEC) and spectrophotometry (SPM)
It is based on the property of colored solutions to absorb non-monochromatic (FEC) or monochromatic (SPM) light in the visible region of the spectrum.
Obtaining colored solutions;
Measurement of optical density (D), which characterizes the absorption of electromagnetic radiation by a solution containing the analyte;
Carrying out calculations based on the basic law of light absorption using a calibration graph, specific absorption coefficient, and standard sample solution.
When determining drugs containing phenolic hydroxyl by these methods, colored compounds are obtained based on complexation reactions with iron (III) ions, azo coupling with diazonium salts and the formation of indophenol dye.
Lecture No. 14
Topic: “Qualitative reactions to functional groups”
1) Hydroxyl (alcohol, phenolic)
2) Carbonyl (aldehyde, carboxyl, ester)
3) Primary aromatic amino group, tertiary amino group (tertiary nitrogen)
4) Imide, sulfamide.
Functional groups (FG) are individual atoms or groups of atoms associated with a carbon radical, which, due to their characteristic properties, can be used to define medicinal substances.
I. Alcohol hydroxyl. Alr-OH is a hydroxyl bonded to an aliphatic hydrocarbon radical.
Based on alcohol hydroxyl, the following reactions are used in the analysis of drugs containing it:
Esterification (formation of esters with acids or their anhydrides);
Oxidation to aldehydes, and in some cases to acids;
Complexation with copper (II) ions in an alkaline medium.
1.Esterification reaction in the presence of water-removing agents with acids or their anhydrides. Based on the property of alcohols to form esters. In the case of low molecular weight compounds, esters are detected by smell; when analyzing drugs with high molecular weight, by melting point.
The esterification reaction is a pharmacopeia for ethyl alcohol.
CH 3 CH 2 OH + CH 3 COOH H2SO4→ CH 3 CH 2 OCOCH 3
2. Oxidation reaction, based on the property of alcohols to oxidize to aldehydes, which are detected by smell. Various oxidizing agents are used as reagents: potassium permanganate, potassium bichromate, potassium hexacyanoferrate (III), etc. Potassium permanganate has the greatest analytical value, which, when reduced, changes the oxidation state from +7 to + 2 and becomes discolored, i.e. makes the reaction effective.

Complexation reaction, based on the property of alcohols to form colored complex compounds with copper (II) sulfate in an alkaline environment.

It is used to identify polyhydric alcohol - glycerol and ephedrine hydrochloride, in which, in addition to the alcohol hydroxyl, a secondary amine group also participates in complex formation.
Phenolic hydroxyl is a hydroxyl bound to an aromatic radical. It contains drug group phenols (phenol, resorcinol); phenolic acids and their derivatives (salicylic acid, phenyl salicylate, salicylamide, oxafenamide); phenanthrene isoquinoline derivatives (morphine hydrochloride, apomorphine); sinestrol, adrenaline, mezaton, etc.
The chemical properties of compounds containing phenolic hydroxyl are determined by the interaction of an electron pair with the π electrons of the aromatic ring. This interaction leads to a shift in the electron density from the OH - group to the ring, disruption of the uniform distribution of electrons in it, and the creation of an excess negative charge in the ortho and para positions. The hydrogen atom of the hydroxy group ionizes and gives phenols weak acidic properties, which, however, are rarely used in analysis. The reactions of electrophilic substitution of hydrogens in the o- and n-positions of the aromatic ring are of greatest importance.
Based on the properties of phenolic hydroxyl, the following reactions are used:
Complexation;
Bromination;
Azose combinations;
Oxidation;
Formation of indophenol dye;
Condensation
1. Complexation reactions phenolic hydroxyl with iron (III) ions. It is based on the properties of phenolic hydroxyl to form soluble complex compounds, often colored blue (phenol) or violet (resorcinol, salicylic acid), less often red (PAS - sodium) and green (quinosol).
The composition of the complex, and, consequently, their color is determined by the amount of phenolic hydroxyls (phenol - blue, resorcinol - purple), and the influence of other functional groups.

2. Bromination reaction aromatic ring. It is based on the electrophilic substitution of hydrogen in the o- and n-position with bromine to form an insoluble bromine derivative.
 Basic rules for bromination
Basic rules for bromination
Bromine replaces hydrogen in the o- and n-positions relative to the phenolic hydroxyl (the most efficient n-position):
 - if there are substituents in the o- or n-positions of the aromatic ring, fewer bromine atoms enter into the reaction:
- if there are substituents in the o- or n-positions of the aromatic ring, fewer bromine atoms enter into the reaction:
 - if there is a carboxyl group in the o- or n- positions, then in the presence of excess bromine, decarboxylation occurs and the formation of a tribromo derivative:
- if there is a carboxyl group in the o- or n- positions, then in the presence of excess bromine, decarboxylation occurs and the formation of a tribromo derivative:
If the substituent is in the m-position, then it does not interfere with the formation of the tribromo derivative:

If a compound contains two phenolic hydroxyls in the m-position, then as a result of their consistent orientation, a tribromo derivative is formed

if two hydroxyl groups are located in the o- or n-position to each other, then they act inconsistently: bromination does not occur:

3.Azo coupling reaction phenols with a diazo reagent (diazotized sulfanilic acid) to form an azo dye, colored orange-red.

4. Oxidation reaction. Phenols can be oxidized to various compounds, but most often to quinones, colored pink or, less commonly, yellow.


5. Reaction of formation of indophenol dye. It is based on the oxidation of phenols to quinones, which, when condensed with ammonia or amino derivatives and excess phenol, form an indophenol dye, colored violet.


6. Condensation reaction with aldehydes or acid anhydrides:
With formaldehyde in the presence of concentrated sulfuric acid to form an aurinic (arylmethane) dye colored red.
The reaction is pharmacopoeial for salicylic acid.

Concentrated sulfuric acid plays the role of a water-removing agent in the first stage of the reaction, and acts as an oxidizing agent in the second.
Chemistry of the reaction to resorcinol

Monohydric phenols (arenols). Nomenclature. Isomerism. Methods of obtaining. Physical properties and structure. Chemical properties: acidity, formation of phenolates, ethers and esters; nucleophilic substitution of hydroxyl group; reactions with electrophilic reagents (halogenation, nitration, nitrosation, azo coupling, sulfonation, acylation and alkylation); interaction with formaldehyde, phenol-formaldehyde resins; oxidation and hydrogenation reactions.
Diatomic phenols (arenediols): pyrocatechol, resorcinol, hydroquinone. Preparation methods, properties and applications.
Trihydric phenols (arenetriols): pyrogallol, hydroxyhydroquinone, phloroglucinol. Preparation methods, properties and applications.
Hydroxyl derivatives of arenes
Phenols are derivatives of aromatic hydrocarbons in which one or more hydroxyl groups are directly attached to the benzene ring.
Depending on the number of hydroxyl groups in the nucleus, one-, two- and triatomic phenols are distinguished.
Trivial names are often used to name phenols (phenol, cresols, pyrocatechol, resorcinol, hydroquinone, pyrogallol, hydroxyhydroquinone, phloroglucinol).
Substituted phenols are referred to as phenol derivatives or hydroxy derivatives of the corresponding aromatic hydrocarbon.
Monohydric phenols (arenols) Ar-OH
ortho-cresol meta-cresol para-cresol
2-methylphenol 3-methylphenol 4-methylphenol
2-hydroxytoluene 3-hydroxytoluene 4-hydroxytoluene
In the aromatic series there are also compounds with a hydroxyl group in the side chain - the so-called aromatic alcohols.
The properties of the hydroxyl group in aromatic alcohols do not differ from the properties of aliphatic alcohols.
Diatomic phenols (arenediols)

pyrocatechin resorcinol hydroquinone
1,2-dihydroxybenzene 1,3-dihydroxybenzene 1,4-dihydroxybenzene
Trihydric phenols (arenetriols)

pyrogallol hydroxyhydroquinone phloroglucinol
1,2,3-trihydroxybenzene 1,2,4-trihydroxybenzene 1,3,5-trihydroxybenzene
Monohydric phenols
Methods of obtaining
A natural source of phenol and its homologues is coal, during dry distillation of which coal tar is formed. When the resin is distilled, a “carbolic oil” fraction (t 0 160-230 0 C) containing phenol and cresols is obtained.
1. Fusion of salts of aromatic sulfonic acids with alkalis
The reaction underlies industrial methods for the production of phenols.
The reaction consists of heating benzenesulfonic acid with solid alkali (NaOH, KOH) at a temperature of 250-300 0 C:
The reaction proceeds by the mechanism of nucleophilic substitution S N 2 aroma(attachment-detachment).
The presence of electron-withdrawing substituents in ortho and para positions relative to the site of substitution facilitates the nucleophilic substitution reaction.
2. Hydrolysis of aryl halides
Aryl halides, which do not contain activating electron-withdrawing substituents, react under very harsh conditions.
Thus, chlorobenzene is hydrolyzed to form phenol by the action of concentrated alkali at a temperature of 350-400 0 C and a high pressure of 30 MPa, or in the presence of catalysts - copper salts and high temperature:

The reaction proceeds by the mechanism of nucleophilic substitution (elimination-addition) (aryne or kine mechanism).
The presence of electron-withdrawing substituents in the ortho and para positions relative to the halogen significantly facilitates the hydrolysis reaction.
Thus, para-nitrochlorobenzene is capable of replacing chlorine with hydroxyl by conventional heating with an alkali solution at atmospheric pressure:
 para-nitrochlorobenzene para-nitrophenol
para-nitrochlorobenzene para-nitrophenol
The reaction proceeds according to the mechanism S N 2
aroma(attachment-detachment).
3. Preparation of phenol from cumene (cumene method)
Synthesis based on cumene is of industrial importance and is valuable because it allows one to simultaneously obtain two technically important products (phenol and acetone) from cheap raw materials (oil, petroleum cracking gases).
Cumene (isopropylbenzene), when oxidized by atmospheric oxygen, turns into hydroperoxide, which, under the action of an aqueous acid solution, decomposes to form phenol and acetone: 
hydroperoxide phenol acetone
4. Hydroxylation of arenes
To directly introduce a hydroxyl group into the benzene ring, hydrogen peroxide is used in the presence of catalysts (iron (I) or copper (I) salts):
5. Oxidative decarboxylation of carboxylic acids
Phenols are obtained from aromatic acids by passing water vapor and air into the reactor at a temperature of 200-300 0 C in the presence of copper salts (P):
6. Preparation from diazonium salts
When arendiazonium salts are heated in aqueous solutions, nitrogen is released to produce phenols: 
Physical properties of phenols
The simplest phenols under normal conditions are low-melting, colorless crystalline substances with a characteristic odor.
Phenols are slightly soluble in water, but highly soluble in organic solvents. When stored in air they darken due to oxidation processes.
They are toxic substances and cause skin burns.
Electronic structure of phenol
The structure and distribution of electron density in a phenol molecule can be depicted by the following diagram:

The hydroxyl group is a substituent of the 1st kind, i.e. electron-donating substituent.
This is due to the fact that one of the lone electron pairs of the hydroxyl oxygen atom enters into p,π-conjugation with the π-system of the benzene ring, exhibiting the +M effect.
On the other hand, the hydroxyl group, due to the greater electronegativity of oxygen, exhibits the –I effect.
However, the +M effect in phenols is much stronger than the oppositely directed –I effect (+M > -I).
The result of the coupling effect is:
1) an increase in the polarity of the O-H bond, leading to an increase in the acidic properties of phenols compared to alcohols;
2) due to conjugation, the C-OH bond in phenols becomes shorter and stronger in comparison with alcohols, since it is partially double in nature. Therefore, OH group substitution reactions are difficult;
3) an increase in electron density on carbon atoms in the ortho- and para-positions of the benzene ring facilitates the reactions of electrophilic substitution of hydrogen atoms in these positions.

Chemical properties of phenols
The chemical properties of phenols are determined by the presence of a hydroxyl group and a benzene ring in the molecule.
1. Reactions on the hydroxyl group
1. Acid properties
Phenols are weak OH-acids, but much stronger than alkanols. Acidity constant rK A phenol is equal to 10.
The higher acidity of phenol is explained by two factors:
1) greater polarity of the O-H bond in phenols, as a result of which the hydrogen atom of the hydroxyl group acquires greater mobility and can be eliminated in the form of a proton to form phenolate ion;
2) The phenolate ion is mesomerically stabilized due to the conjugation of the oxygen lone pair with the benzene ring, i.e. the negative charge on the oxygen atom of the phenolate ion is significantly delocalized:

None of these boundary structures alone describes the actual state of the molecule, but their use allows us to explain many reactions.
Electron-withdrawing substituents increase the acidic properties of phenol.
By drawing electron density from the benzene nucleus toward themselves, they enhance p,π-conjugation (+M-effect), thereby increasing the polarization of the O-H bond and increasing the mobility of the hydrogen atom of the hydroxyl group.
For example:
 phenol 2-nitrophenol 2,4-dinitrophenol picric acid
phenol 2-nitrophenol 2,4-dinitrophenol picric acid
рК а 9.98 7.23 4.03 0.20
Electron-donating substituents reduce the acidity of phenols.
1. Substitution of phenolic hydroxyl with halogen
The hydroxyl group in phenols is very difficult to replace with halogen.
When phenol reacts with phosphorus pentachloride PCl 5, the main product is triphenyl phosphate and only small amounts of chlorobenzene are formed:
Triphenylphosphate chlorobenzene
The presence of electron-withdrawing substituents in the ortho- and para-positions relative to the hydroxyl greatly facilitates the reactions of nucleophilic substitution of the OH group.
Thus, picric acid under the same conditions is easily converted into 2,4,6-trinitrochlorobenzene (picryl chloride):  picric acid picryl chloride
picric acid picryl chloride
2. Interaction with ammonia
When interacting with ammonia at elevated temperature and pressure in the presence of an aluminum chloride catalyst, the OH group is replaced by an NH 2 group to form aniline:
 phenol aniline
phenol aniline
3. Phenol reduction
When phenol is reduced with lithium aluminum hydride, benzene is formed:
3. Reactions involving the benzene ring
1. Electrophilic substitution reactions in the benzene ring
The hydroxyl group is a substituent of the 1st kind, therefore, electrophilic substitution reactions in the benzene ring occur with phenols much more easily than with benzene, and the substituents are directed to the ortho and para positions.

1) Halogenation reactions
Phenol easily reacts with bromine water at room temperature to form a white precipitate of 2,4,6-tribromophenol:

2,4,6-tribromophenol
This reaction is qualitative for phenols.
Phenol chlorination occurs easily:

2) Nitration reactions
Phenol is easily nitrated with dilute nitric acid at a temperature of 0 0 C to form a mixture of ortho and para isomers with a predominance of the ortho isomer: 
ortho- and para-nitrophenols
Isomeric nitrophenols are easily separated due to the fact that only the ortho isomer is volatile with water vapor.
The greater volatility of ortho-nitrophenols is explained by the formation of intramolecular hydrogen bonds, while the para-isomer forms intermolecular hydrogen bonds: 
When concentrated nitric acid is used, 2,4,6-trinitrophenol (picric acid) is formed:
 picric acid
picric acid
3) Sulfonation reactions
Phenol is easily sulfonated at room temperature with concentrated sulfuric acid to form an ortho isomer, which at temperatures above 100 0 C rearranges into a para isomer:

4) Alkylation reactions
Phenols easily undergo alkylation reactions.
Haloalkanes, alkanols and alkenes are used as alkylating agents in the presence of protic acids (H 2 SO 4, H 3 PO 4) or Lewis acids (AlCl 3, BF 3): 

5) Acylation reactions
Acylation of phenols occurs easily under the action of halogen anhydrides or carboxylic acid anhydrides in the presence of Lewis acids: 
6) Nitrosation reactions
Nitrosophenols are obtained by direct nitrosation of phenols:

para-cresol ortho-nitroso-para-cresol
7) Azo coupling reactions
Combination with phenols leads to slightly alkaline environment, since the phenolate ion is much more active than phenol itself:

8) Condensation reactions
Phenols are such active components in electrophilic substitution reactions that they interact with very weak electrophiles - aldehydes and ketones in the presence of acids and bases.
Condensation with formaldehyde
Formaldehyde most easily enters into condensation reactions.
If the condensation reaction of phenol with formaldehyde is carried out under mild conditions, it is possible to isolate ortho- and para-hydroxymethylphenols: Individual representatives
Phenol– crystalline substance with m.p. 43°C, has a characteristic pungent odor, causes burns on the skin. This is one of the first antiseptics used in medicine. It is used in large quantities to produce plastics (condensation with formaldehyde), medicines (salicylic acid and its derivatives), dyes, explosives (picric acid).
Phenol methyl ether – anisole– used to produce aromatic substances and dyes.
Phenol ethyl ether – phenetol.
Cresols (methylphenols) used in the production of plastics, dyes, and disinfectants.
Phenolic hydroxyl is a hydroxyl associated with an aromatic ring.
1. Acid-base properties are due to the presence of a mobile hydrogen atom in phenolic hydroxyl. The electron pair of the hydroxyl is shifted towards the aromatic ring, therefore the acidic properties are stronger than those of alcohols. So pKa of carbonic acid = 6.35, and pKa of phenol = 9.89.
Phenols dissolve in aqueous solutions of alkalis to form phenolates (phenoxides):
However, the acidic nature of phenols is expressed so insignificantly that even such a weak acid as carbonic acid displaces phenols from their salts:
Therefore, phenols, dissolving in alkalis, cannot dissolve in carbonates, because the carbonic acid released in this case immediately decomposes the phenolate:
This property of phenols distinguishes them from carboxylic acids.
As the temperature increases, the reaction proceeds in the forward direction. Alkali metal phenolates, as salts of strong bases and weak acids, are partially hydrolyzed in aqueous solutions, therefore solutions of phenolates have an alkaline reaction.
2. Esterification reaction (similar to alcohol hydroxyl).
The formation of ethers is the reaction of phenolates and alkyl halides (or alkyl sulfates).
C 6 H 5 ONa+JCH 3 ®C 6 H 5 OCH 3 +NaJ
Esters are formed by the reaction of sodium phenolates with anhydrides (or acid chlorides).
3. Redox properties.
Phenols exhibit strong reducing properties and are very easily oxidized even by weak oxidizing agents, resulting in the formation of colored compounds with a quinoid structure.
 | [O] – CaOCl 2, H 2 O 2, Cl 2, Br 2 |
An example of an oxidation reaction is the formation of an indophenol dye: the resulting quinone, upon interaction with NH 3, is converted into a quinone imine, which reacts with unreacted phenol. In the presence of ammonia, indophenol is formed, colored blue.

quinoneimine indophenol
n- benzoquinoneimine
A type of indophenol reaction is the Lieberman nitroso reaction, which is characteristic of those phenols in which no substituents at ortho and para positions.
When exposed to sodium nitrite in an acidic environment, it forms n-nitrosophenol, isomerizing to monooxime n-benzoquinone, which then reacts with excess phenol in an acidic environment to give indophenol.

A color is observed that changes when an alkali solution is added:
phenol – dark green, turning into cherry red;
thymol – blue-green, turning purple;
resorcinol – violet-black, turning into violet;
hexestrol (sinestrol) – red-violet, turning into cherry.
4. Complexation reaction with iron ions.
Depending on the amount of phenolic hydroxyls, the presence of other functional groups in the molecule, their relative position, the pH of the environment, and temperature, complex compounds of various compositions and colors are formed (with the exception of thymol).



Complexes are colored:
phenol – blue color;
resorcinol – blue-violet color;
salicylic acid – blue-violet or red-violet color;
osalmid (oxaphenamide) – red-violet color;
sodium para-aminosalicylate – red-violet color;
quinosol – bluish-green color.
The reaction is pharmacopoeial for most phenolic compounds.
5. Electrophilic substitution reactions – SE of a hydrogen atom in the aromatic ring (bromination, condensation with aldehydes, combination with diazonium salts, nitration, nitrosation, iodination, etc.). The ability of phenols to enter into electrophilic substitution reactions is explained by the interaction of the lone electron pair of the oxygen atom with the π-electrons of the benzene ring. The electron density shifts towards the aromatic ring. The greatest excess of electron density is observed at carbon atoms in O- And n- positions relative to the phenolic hydroxyl (type I orientant).
5.1. Halogenation reaction (bromination and iodination).
5.1.1. When interacting with bromine water, white or yellow precipitates of bromine derivatives are formed.

When there is an excess of bromine, oxidation occurs:

The bromination reaction of phenols depends on the nature and position of the substituents.
Iodization occurs similarly, for example:

5.1.2. If there are substituents in O- And n- positions of the aromatic ring, unsubstituted hydrogen atoms of the aromatic ring react.

5.1.3. If in O- And n- positions in relation to the phenolic hydroxyl there is a carboxyl group, then under the action of excess bromine decarboxylation occurs:

5.1.4. If a compound contains two phenolic hydroxyls in m- position, then under the action of bromine tribromo derivatives are formed (consistent orientation):

5.1.5. If two hydroxyl groups are located relative to each other in O- or n- positions, then the bromination reaction does not occur (inconsistent orientation)

5.2. Condensation reactions
5.2.1. With aldehydes.
An example of the condensation of phenols with aldehydes is the reaction with Marquis reagent. When phenols are heated with a solution of formaldehyde in the presence of concentrated H 2 SO 4, colorless condensation products are formed, the oxidation of which produces intensely colored compounds of a quinoid structure. Sulfuric acid plays the role of a dehydrating, condensing and oxidizing agent in this reaction.

5.2.2. The reaction of phenols with chloroform (CHCl 3) to form aurine dyes.
When phenols are heated with CHCl 3 in an alkaline environment, aurines– triphenylmethane dyes:

Aurines are colored:
phenol – yellow color;
thymol – yellow color turning to purple;
resorcinol – red-violet color.
5.2.3. With acid anhydrides.
A. The reaction of fluorescein formation (condensation of resorcinol with phthalic anhydride).


B. Reaction of formation of phenolphthalein (condensation of phenol with phthalic anhydride).

With a large excess of alkali, a trisubstituted sodium salt is formed.
The condensation of thymol with phthalic anhydride proceeds similarly to the reaction of the formation of phenolphthalein; thymolphthalein is formed, which has a blue color in an alkaline medium.
5.3. Nitration reaction
Phenols react with dilute nitric acid (HNO 3) and form ortho- and para-nitro derivatives. The addition of sodium hydroxide solution enhances the color due to the formation of a well-dissociated salt.

5.4. The reaction of azo coupling of phenols with diazonium salt in an alkaline medium.
When phenols react with diazonium salt at pH 9-10, azo dyes are formed, colored yellow-orange or red. The azo coupling reaction occurs in the ortho and para positions relative to the phenolic hydroxyl. Diazotized sulfanilic acid is usually used as a diazo reagent.


Introduction
Most drugs used in medical practice are organic compounds. The identity of such substances is confirmed by reactions to functional groups.
A functional group is a reactive atom, group of atoms, or reaction center in a molecule of an organic compound.
The general principle of functional analysis is the use of characteristic reactions for the groups to be determined. The reaction must not only be as specific as possible, but also sufficiently rapid, and it must involve a reactant or product of the reaction that is easily detectable.

Identification of alcohol hydroxyl
Alcohols - These are derivatives of hydrocarbons, in the molecules of which one or more hydrogen atoms are replaced by hydroxyl groups. In general, an alcohol molecule can be represented as ROH.
Ester formation reaction
Alcohols form esters with organic acids or acid anhydrides in the presence of water-removing agents (for example, concentrated sulfuric acid). Esters obtained from low molecular weight alcohols have a characteristic odor, and esters based on high molecular weight alcohols are crystalline substances with a clear melting point.
Methodology. To 1 ml of ethanol add 5 drops of glacial acetic acid, 0.5 ml of concentrated sulfuric acid and carefully heat; a characteristic odor of ethyl acetate (fresh apples) is detected.

Oxidation reaction of alcohols to aldehydes
The resulting aldehydes are detected by smell. Potassium hexacyano-(III)-ferrate, potassium permanganate, potassium dichromate, etc. are used as oxidizing agents.
Methodology. Place 2 drops of ethanol, 1 drop of 10% sulfuric acid solution and 2 drops of 10% potassium dichromate solution into the first test tube. The resulting solution has orange color. Heat it over a flame until the solution begins to acquire bluish-green color(at the same time, a characteristic smell of acetaldehyde is felt, reminiscent of the smell of Antonov apples). Add 1 drop of the resulting solution to a second test tube with 3 drops of fuchsinsulfurous acid. Appears pink-violet color.
Reaction of formation of complex compounds
Polyhydric alcohols form blue complex compounds with copper sulfate in an alkaline medium (with Fehling's reagent).
Methodology. To 0.5 ml of glycerin add 5 drops of solutions of sodium hydroxide and copper (II) sulfate, intense blue coloring.

Identification of phenolic hydroxyl
Reaction with iron (111) chloride
A characteristic qualitative reaction to phenols is the reaction with iron (III) chloride. Depending on the amount of phenolic hydroxyls, the presence of other functional groups in the phenol molecule, their relative position, the pH of the environment, and temperature, complex compounds of various compositions and colors are formed.
Methodology. To 0.01 g of the drug dissolved in 1 ml of water (for phenol, resorcinol), add 2 drops of iron (III) chloride solution - characteristic coloring is observed (Table 1).
Table 1. Staining of preparation complexes with iron (III) chloride
|
A drug |
Solvent |
Coloring of the complex |
|
Purple |
||
|
Resorcinol |
Blue-violet |
|
|
Adrenaline hydrochloride |
Emerald green, turning from adding one drop of ammonia solution to cherry red, and then orange-red. |
|
|
Morphine hydrochloride |
Blue, disappearing with the addition of diluted acetic or hydrochloric acids |
|
|
Paracetamol |
Blue-violet |
|
|
Pyridoxine hydrochloride |
Red, disappearing with the addition of dilute hydrochloric acid and not disappearing with dilute acetic acid. |
|
|
Salicylic acid and sodium salicylate |
Blue-violet, does not disappear with the addition of a few drops of diluted hydrochloric or acetic acid. |
|
|
Phenyl salicylate |
purple, disappearing from the addition of diluted hydrochloric or acetic acids and turning into blood red by adding 1-2 drops of ammonia solution. |
Using an ammonia solution, you can distinguish phenol from resorcinol. The color of the resorcinol complex with iron after adding the reagent changes to brownish yellow.